|
Let the Devil Wear Black | James F Linden. The true story of the Courtaulds Cancer-Gas Scandal. |
Resources - Thermodynamics
Why the Laws of Thermodynamics are so important.
Chapter 6
In a number of places in the book, there are references to the Second Law of Thermodynamics being violated. So, what is the importance of this?
The name ‘Thermodynamics’ implies movement of heat or, as we would look at it today, the transfer of energy. So, what are the laws of thermodynamics? The first two — that is to say, the first and second laws — appeared as attempts to make steam engines more efficient during the industrial revolution and the rest is history, as they say.
The first law dates to the mid 1800s with the second law following shortly afterwards. The third law follows in the early 1900s and abandoning the conceit that the numbering of laws should in some way be related to the temporal order in which they are discovered, late in the last decade of the 1900s, the zeroth law of thermodynamics came into being — its naming possibly being an ironic statement about the second law.
First Law:The First Law of Thermodynamics states that energy can neither be created nor destroyed. This also extends to mass when described as a form of energy which is in effect, energy in a form that moves slower than the speed of light.
Second Law:The second law of thermodynamics states that in a closed system, the level of disorder only ever increases with time.
The level of disorder is called ‘entropy’ and, like beauty, is in the eye of the beholder. For instance, I can order my desk by putting things into certain places that signify things to me such as things that I have done, things that I have yet to do, things that I am referring to and so on. I can make this very organised but my desk is not a closed system. I am using energy to perform this organisational activity which creates disorder elsewhere — the food I eat contains energy which comes from the sun and so on.
However, my wife might think that my desk is a mess and come along and put all of those ‘messy’ piles of things in one ‘neat’ pile that gives the desk a more pleasing, ‘spacious’ look to it — this, of course, taking more energy to do. However, all of the organisation that I put there has been destroyed by the process, therefore the entropy from my perspective has increased. I could put it all back the way that it was but that would require still more energy and so on. Overall, the level of entropy only ever increases in a closed system and when you have the Earth and the Sun, you have what approximates for most purposes, a close system.
The fact that entropy only ever increases also means that you cannot travel backwards in time. Doing so would break the second law of thermodynamics so it can’t happen.
Also, it means that heat energy only ever flows from hot to cold — even in a refrigerator, heat flows from hot to cold.
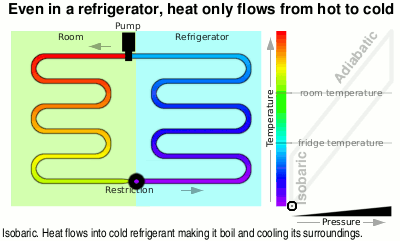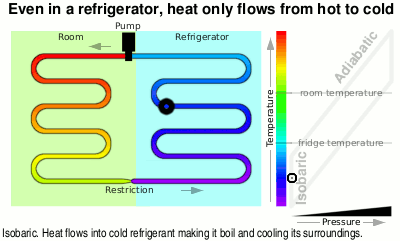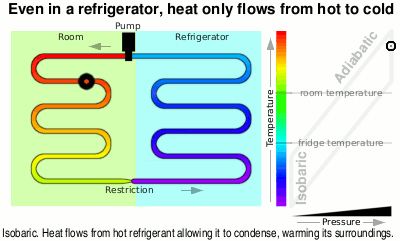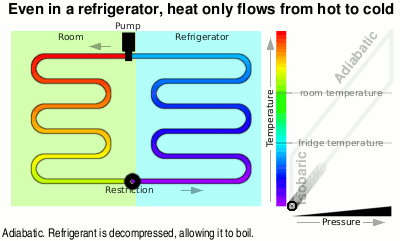
The diagram is simplified but shows what is going on — the temperature scale is slightly different in proportion, for instance, as the refrigerant undergoes a phase change between liquid and vapour.
You can see in the diagram that the liquid at just above room temperature goes through a restriction which allows the pressure to drop and therefore it is now able to boil. When it boils, it takes on heat energy from the surroundings, cooling them down — heat flows from hot(ter) to cold(er).
At the top of the diagram, the vapour goes through a pump and like air that you are compressing in a bicycle pump, it gets hotter.
Next it is fed through the large heat exchanger on the back of your refrigerator and as it cools, it condenses back to liquid again, giving off even more heat, ready to pass through the restriction, starting the cycle again.
The fact that thermal energy only passes from hot to cold means that the colder things warm up and this will happen until everything is the same temperature. This means that eventually, the universe — which is a closed system — will become completely disorganised and there will be no place where there is less energy so without any energy flow, the universe will die.
Third Law:The third law states that the entropy of a system approaches a constant value as the temperature approaches absolute zero asymptotically — it never gets there. A perfect crystal at absolute zero has an entropy of zero.
Zeroth Law:When the temperature of two bodies is the same, there is no heat transfer.
So:In the context of the book, the references to the second law of thermodynamics are in terms of time travel being an impossibility, therefore, for evidence to appear out–of–sequence, it must be the result of fraud or some other, similar process.
“I’ve got a tape recorder with me.” - “Can it record without being seen?”
Copyright © 1994-2023 James F Linden. All Rights Reserved.