|
Let the Devil Wear Black | James F Linden. The true story of the Courtaulds Cancer-Gas Scandal. |
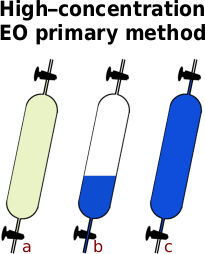
Resources - Primary EO Method
High-concentration ethylene oxide analysis.
Chapter 19
This is beautiful because it is so simple.
The vent gasses from the reactor and one or two other sources including the padded storage tanks which were vented whenever a tanker was unloaded, had high concentrations of Ethylene Oxide in them and were the source of the reason why I had to re–analyse them, just to make sure that they were correct.
Once it was pointed out to the Environment Agency (EA) that the levels of EO at the perimeter fence were, as stated by ‘Deryck’ during 1993, in the order of ‘ten times the legal limit’, a response to the EA from ‘Deryck’ stated that the original analysis was performed ‘on a very old and inaccurate packed column gas chromatograph ’ which he alleged had ‘poor resolution’ and then claimed that the analysis was repeated on a GCMS which enabled the job to be performed ‘more accurately’.
The samples concerned were of the order of 30% EO with the only other component being nitrogen gas. There was no reason to use a chromatograph of any sort to separate anything out because there were no other chemicals in there, just the two.
Additionally, the concentration of EO was so high that it was easily detectable using a method other than chromatography — chromatography really only starts to be useful at levels below a few percent in samples like this. As though that was not enough, chromatography is a secondary method — you need to calibrate the machine using standard samples — and therefore is not as accurate as the method I actually used, which is a primary method — it is measured directly and needs no standards. In fact, this method is so robust that even if you are careless with it, you will still have an error of around a quarter of a per cent, whereas chromatography is around five and two per cent for packed columns and capillary columns respectively — the mass–spectrometer of the GCMS only being a detector and not contributing to the accuracy — GCMS is still a secondary method.
So, how does it work?
It works on the following points:
- EO is very soluble in water;
- there are only two components, the other (nitrogen) is not soluble in water to an extent that would interfere with the analysis;
- the concentration of EO in the sample is high; and,
- the pressures involved in the plant are low — no more than 2psig.
This is what you do...
- Go on the plant with an evacuated 500ml sample tube.
- Flush the sample point by opening the valve.
- Put the sample tube in place on the silicone tube and open the tap to let the sample gas flow in.
- Once you have the sample, close the tap and then the valve — optionally, you can open the other tap and let the sample gas blow through it for a few seconds before closing the taps and the valve.
- Take it back to the lab and let it cool down.
- a Weigh the sample tube on a balance to the nearest gramme (weight ‘T’).
- Put the end of the sample tube into a tub of running water and just crack open the tap to let out any pressure so that the pressure in the tube is at atmospheric pressure, ie the end of the tube is only just below the surface — arguably you should get the tare weight at this point rather than the last but the difference in weight is so small and the tube would now need drying.
- Push the end of the tube down into the water so that some water enters the sample tube and the EO will immediately start to dissolve into it, reducing the pressure and sucking more water in there.
- Periodically close the tap and shake the water around in the tube, then immersing the end before opening the tap to let more water in. When no more water goes in, the EO is all absorbed and the only gas left in the tube is nitrogen.
- Raise the sample tube with the tap open until the level of the water on the inside is the same as it is on the outside and close the tap.
- b Dry off the tube and weigh it on a balance to the nearest gramme (weight ‘N’).
- Finally, open up both taps with the tube underwater and fill it completely with water. Close at least one of the taps so that water doesn’t run out — if you close both and then forget about it once you have finished, the water inside would warm up and expand, the resulting pressure can be enough to break the sample tube.
- c Dry off the tube and weigh the sample tube on a balance to the nearest gramme (weight ‘G’).
Now, take weight ‘T’ off the other two weights and then divide one by the other like so...
Percentage EO = (N - T) / (G - T) x 100
The maths is easy, the rest of it is quick and as for errors, if you make sure that the pressure before you add the water is the same as the pressure in the lab, you might get an error of around 1 gramme or one part in 500 aka 0.2% which by far outshines any chromatographic methods — even the primary method ‘Janak’ when considering the volumes involved.
Then, I called the police.
Copyright © 1994-2023 James F Linden. All Rights Reserved.